Introduction: MPN are a heterogeneous group of chronic hematological malignancies often resulting from a combination of a driver gene mutation (JAK2, MPL or CALR) and a variety of somatic mutations harboring diverse prognosis values. A subset of MPN patients carry somatic mutations in the hematopoietic transcription factor NFE2 (nuclear factor erythroid 2) resulting in a functionally enhanced truncated form of NFE2 (Jutzi JS et al., JEM, 2013). Moreover, epigenetically induced overexpression of NFE2 has recently been reported in the majority of MPN patients (Peeken JC et al., Blood, 2018). In transgenic murine models, NFE2 overexpression results in an MPN phenotype (thrombocytosis, leukocytosis, EPO-independent colony formation, characteristic bone marrow histology and expansion of stem and progenitor compartments) and has recently been shown to predispose to the acquisition of additional genetic abnormalities and subsequent leukemic transformation (Kaufmann KB et al., JEM, 2012) (Jutzi JS et al., Blood, 2019). However, clinical impact of NFE2 mutations in MPN patients remains unknown. The aim of this study was to evaluate the phenotypic characteristics and prognostic impact of NFE2 somatic mutations in a large mono-centric cohort of MPN patients.
Methods: A total of 1243 consecutive patients were diagnosed with MPN according to WHO criteria and followed in our hospital between January 2011 and May 2020. This study included 707 of them in whom a next-generation sequencing (NGS) molecular analysis targeting 36 myeloid genes was performed at diagnosis and/or during follow-up. Clinical and biological characteristics at time of diagnosis and follow-up were collected from medical charts and electronic medical records. Statistical analyses were performed using the STATA software (STATA 15.0 Corporation, College Station, TX).
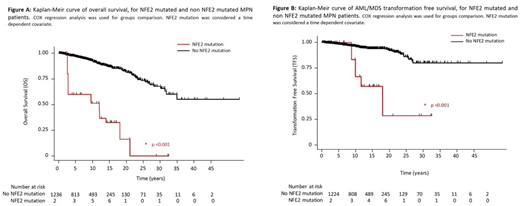
Results: In our cohort, 411 patients presented with polycythemia vera (PV), 577 with essential thrombocythemia (ET), 184 with primary or pre-fibrotic myelofibrosis (PMF), 59 with unclassified MPN and 12 with MDS/MPN. Median age at diagnosis was 51 years [40-63]. 73.1% patients had a JAK2V617F mutation, 14.1% a CALR mutation and 3.3% a MPL mutation. Overall, 64 (9.05%) patients harbored a NFE2 mutation with a variant allelic frequency (VAF) ≥ 0.5% and 36 had a VAF ≥ 5%, the latest were considered as NFE2 mutated for the rest of the study as VAF <5% may refer to a minor clone without clinical relevance. NFE2 mutations were present in 7.3%, 5.3% and 3.6% of PV, PMF and ET patients respectively. No significant association between the presence of NFE2 mutation and clinical/molecular MPN characteristics (driver mutation, constitutional symptoms, splenomegaly, blood counts, cytogenetic and other molecular features) was observed using a logistic regression association model. Median follow-up was 103.8 months, IQR [47.2; 175.6]. In terms of response to therapy, 52.8% of patients achieved complete response, complete hematological response or clinical improvement in NFE2 mutated vs 61.7% in non-mutated patients (p= 0.026). Interestingly, presence of a NFE2 mutation (HR 9.92, 95%CI[3.21; 30.64], p< 0.001), age at diagnosis (HR 1.09, 95%CI[1.05; 1.12], p< 0.001), PMF subtype (HR 6.92, 95%CI[2.81; 17.06], p < 0.001) and high-risk mutations (ASXL1, EZH2, SRSF2, IDH1/2 and U2AF1) (HR 2.45, 95%CI[1.14; 5.28], p=0.021) were independently associated with AML/MDS transformation free survival (TFS) in a COX regression multivariate analysis (Figure A). Presence of a NFE2 mutation was also independently associated with overall survival (OS) (HR 9.37, 95%CI [4.18; 21.03], p<0.001) (Figure B). Median TFS were 216.1 months and not reached, while median OS were 144.2 months and not reached for NFE2 mutated and non-mutated patients, respectively. No difference was observed in terms of thrombo-hemorrhagic events (HR 0.73; 95%CI [0.10; 5.21], p=0.752) and secondary myelofibrosis free survivals (HR 0.67; 95%CI [0.09; 4.87], p=0.693).
Conclusion: In this retrospective study we show that presence of NFE2 mutations with a VAF ≥5% is independently associated with an increased risk of leukemic transformation and shorter overall survival. These findings are in line with recently reported animal models and suggest that NFE2 mutations screening should be routinely performed in MPN patients.
Rea:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Benajiba:Gilead Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.